- Community
-
Programs
- Schools
-
Careers
- RN Specialties
- Best RN Jobs and Salaries
- Aesthetic Nurse
- Nursing Informatics
- Nurse Case Manager
- NICU Nurse
- Forensic Nurse
- Labor and Delivery Nurse
- Psychiatric Nurse
- Pediatric Nurse
- Travel Nurse
- Telemetry Nurse
- Dermatology Nurse
- Nurse Practitioner
- Best NP Jobs and Salaries
- Family NP (FNP)
- Pediatric NP
- Neonatal NP
- Oncology NP
- Acute Care NP
- Aesthetic NP
- Women's Health NP
- Adult-Gerontology NP
- Orthopedic NP
- Emergency NP
- Psychiatric-Mental Health NP (PMHNP)
- APRN
- Nurse Educator
- Nurse Administrator
- Certified Nurse Midwife (CNM)
- Clinical Nurse Specialist (CNS)
- Certified Registered Nurse Anesthetist (CRNA)
- Resources
- Education
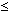 100 mg/day) for at least 3 months after the MI is recommended.
100 mg/day) for at least 3 months after the MI is recommended.
SleeepyRN
1,076 Posts
Hello. I graduated nursing school 3 years ago. We learned that the reference range for patients on Coumadin is 2.0 - 3.0 and maybe 0.5 higher if at especially great risk for a blood clot.
Well, I admitted a patient the other day who had a hx of PE leading to cardiac arrest and ultimately brain anoxia. He was admitted to our rehab facility after a short stay in the hospital d/t aspiration pneumonia and sepsis. He is completely immobile, does not respond to verbal commands nor makes any attempt to move any body part on his own. Upon admission he was on 7.5 mg Coumadin and 60mg lovenox.
Upon viewing his labs, I saw his INR was 2.6 with an H next to it. So I looked at the hospital's reference range. I was surprised to see
0.86 - 1.8.
I remember learning SOMETHING in nursing school about certain individuals reference range falling between those numbers. But I don't remember what. I can't for the life of me figure out why the hospital's INR reference range would be this low, especially for a patient with a hx of DVTs, PE and ultimately cardiac arrest. Thoughts?
I feel I've been away from bedside too long and that I've forgotten SO much. I'm actually reading my nursing books again and listening to lectures.