- Community
-
Programs
- Schools
-
Careers
- RN Specialties
- Best RN Jobs and Salaries
- Aesthetic Nurse
- Nursing Informatics
- Nurse Case Manager
- NICU Nurse
- Forensic Nurse
- Labor and Delivery Nurse
- Psychiatric Nurse
- Pediatric Nurse
- Travel Nurse
- Telemetry Nurse
- Dermatology Nurse
- Nurse Practitioner
- Best NP Jobs and Salaries
- Family NP (FNP)
- Pediatric NP
- Neonatal NP
- Oncology NP
- Acute Care NP
- Aesthetic NP
- Women's Health NP
- Adult-Gerontology NP
- Orthopedic NP
- Emergency NP
- Psychiatric-Mental Health NP (PMHNP)
- APRN
- Nurse Educator
- Nurse Administrator
- Certified Nurse Midwife (CNM)
- Clinical Nurse Specialist (CNS)
- Certified Registered Nurse Anesthetist (CRNA)
- Resources
- Education

lil_ash03
4 Posts
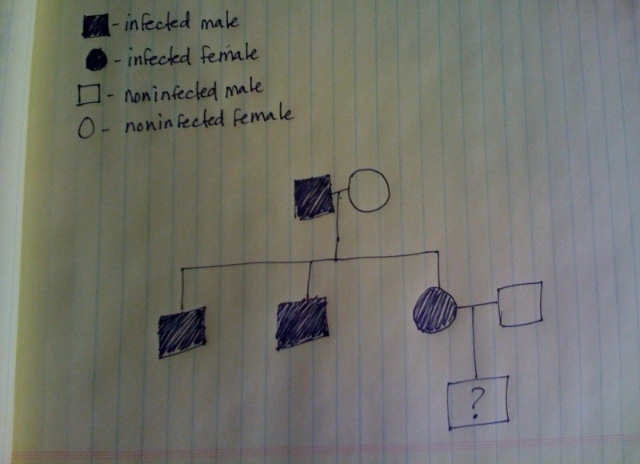
I am getting ready to retake the Teas test at the end of this month. I am trying to find some info on a science question. It was about predicting future offspring..it wasnt a punett square though..the name of it or any info on it would be much greatly appreciated.
thank you in advance,
lil_ash:heartbeat